Home » FAQs
FAQ
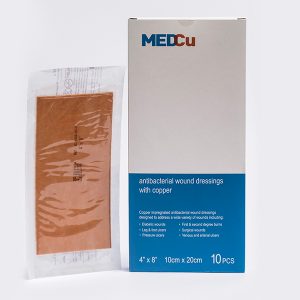
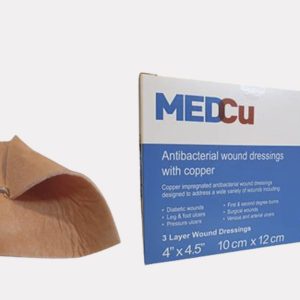
MedCu copper dressings are antibacterial absorbent 2- or 3-layer sterile, soft, non-adherent, single use wound dressings consisting of the following:
- An internal absorbent cellulose and polyester layer containing copper oxide
- One or two external non-adherent nonwoven polypropylene layers containing copper oxide
- Adhesive contour (in some of the models)
Prescription (Rx only) use for the dressings: Partial and full thickness wounds, Pressure ulcers (stage I-IV), Diabetic ulcers, Venous stasis ulcers, Arterial ulcers, 1st and 2nd degree burns, Surgical wounds, Vascular access or peripheral IV sites, Orthopedic external pin sites, and Acute wounds such as lacerations, abrasions, and skin tears.
Cleanse and dry peri-wound area thoroughly before dressing application as per facility protocol.
If necessary, debride necrotic tissue before applying dressing. Hydrate wounds with low or no exudate as per facility protocol. If necessary, treat infection as per facility protocol.
- Choose the appropriate size and form dressing
- Peel-open the sterile pouch from the top side
- Remove aseptically the wound dressing
- If using the MedCu 3-layer wound dressings (orange on both sides), apply either side directly onto the wound. Cover with a secondary dressing and secure with fixation tape or a wrap.
- If using the MedCu 2-layer wound dressings (with one side orange) apply the orange side of the dressing to the wound site. Cover with a secondary dressing and secure with fixation tape or a wrap.
- If using the MedCu wound dressings with adhesive contour, no additional fixation, tape or wrap, are needed
*The clinician should evaluate each wound and patient to determine the best fixation method for the MedCu dressings. The clinician may choose any fixation method per institutional protocols.
Frequency of wound dressing change may vary according to wound exudate and condition and clinician considerations. MedCu copper dressing may be left in place for up to 7 days.
The wound dressings may be used for a maximum of 30 days total, including reapplications
Both copper and silver have wide spectrum antimicrobial properties. Some microorganisms are more sensitive to copper and some more sensitive to silver. The biocidal mechanism is similar in both cases, i.e. non-specific multisite kill mechanisms. Currently in the marketplace there are many “silver dressings” but only one “copper dressing” that have been cleared for clinical use by the FDA and European regulatory bodies. In regards to antimicrobial wound dressings, their antibacterial efficacy depends on several parameters, such as the exact silver or copper compound used, the concentration of the active agent, etc. Most of the silver dressings in the marketplace have been cleared for use many years ago, when the biocidal requirements by the regulatory bodies were lower. It is sure they would have been cleared today, as their antibacterial efficacy is not high. We have compared the biocidal potency of many silver dressings versus MedCu copper dressing using the well accepted quantitative test method – AATCC TM 100 – and found that copper dressings demonstrated much more potent efficacy than almost all silver dressings tested.
We have conducted the following safety studies using independent laboratories and GLP in accordance with the requirements for medical devices and passed all of them successfully:
- Acute systemic toxicity (Mice)
- Wound healing chronic toxicity study (Porcine)
- Skin irritation (Rabbits)
- Intracutaneous reactivity (Rabbits)
- Sensitization (Maximization) (Guinea pigs)
- Cytotoxicity (cell culture)
- Pyrogenicity test (Rabbits)
In addition, we successfully passed the exhaustive extractions, FTIR, GC-MS, ICP-OES, UPLC-MS test performed using GLP, demonstrating the high purity and safety of the dressing.
Based on the above extensive safety studies, MedCu wound dressings were cleared by the FDA and other regulatory bodies to be used clinically.
Currently the dressings are being used clinically in several countries for over 2 years, with hundreds of patients already being treated, without even one adverse reaction recorded.
In contrast to some silver dressings that can cause coloration, copper does not cause any argyria or any skin coloration. Copper oxide impregnated consumer products, such as pillowcases, diapers, medical textiles (sheets, blankets, etc.), socks, etc. are being used for more than 15 years by hundreds of thousands of individuals worldwide without any irritation reports, etc. This is the same exact technology used by MedCu by the sister company Cupron. On the contrary, these products have very good effects on the skin of those using them.
The wound dressing is provided sterile using ETO sterilization method. Sterility is maintained as long as the pouch is not opened or breached. MedCu copper dressings absorb wound exudates, contain copper-oxide to inhibit bacterial growth within the dressing and serves as a *barrier against bacterial penetration.
*Note: Barrier against external contamination is achieved only by the adhesive contour model
MedCu copper dressings should be changed when signs of saturation of the dressing are noticeable or whenever clinician practice and facility protocol dictates
- To change the non-adhesive MedCu dressings, remove the fixation bandages or tape and carefully lift the dressing off the wound. Wet with sterilized saline, if needed, for easier removal of the wound dressing.
- To change the adhesive MedCu dressings, carefully peel the adhesive contour layer and gently lift the dressing off the wound.
For external use only. MedCu copper dressings may be used on infected wounds being managed in accordance with institutional clinical protocols for infection abatement as an adjunct to the standard treatment regimen. Do not use if the package is open or damaged
Sterility is maintained as long as the sterile pouch is not breached. Single use only. Do not resterilize.
The dressings should be stored at room temperature.
MedCu impregnates both layers of the wound dressings, the external nonwoven layer and the internal absorbent layer, with cuprous oxide microparticles. These microparticles are physically embedded in the polymeric matrix during the manufacturing process. These particles do not detach from the polymeric substrate, as demonstrated to the FDA and other regulatory bodies, but these particles serve as a reservoir of copper ions, which are constantly liberated in the presence of skin humidity and wound exudates.
The external wound dressing layer is a non-adherent layer that allows the passage of the wound exudates into the highly absorbent internal layer of the dressing. With the wound exudates bacteria present in the wound are also absorbed to a certain degree. The released copper ions reach a concentration that results in the killing of the bacteria, in the wound dressing itself and with time also in the tissue. We have demonstrated to the FDA that the wound dressing kills more than 4 logs of gram positive and gram negative bacteria immediately and the dressing preserves this capability also after being fully absorbed with wound exudates for 7 days.
As explained above in point 3, we have data for 7 days. Accordingly, the wound dressings are to be used for up to 7 days or until the wound is fully soaked with wound exudate.
The wound dressing can absorb ~ 8-10 times their own weight, depending on the SKU (dressing type). As long as the dressings have not reached their maximum absorbency capacity, the absorbed exudate (and bacteria) are entrapped in the dressing. The dressings are usually changed before reaching 7 days per single use in middle and highly exudating wounds. Nevertheless, we have repeatedly observed that a highly exudating wound will culminate in wetting and even greenish discoloration of the external paddings, but without harm to the skin at the wound edge. The explanation to this is that the dressing actually has two layers of bacterial killing properties: the inner one with higher concentration and the absorbent layer which still has cuprous oxide in bactericidal concentration. We rarely saw skin irritation and maceration even in highly exudating wounds. In addition, it is possible to change the outer padding without changing the copper dressing on the wound, just to receive more outer absorbent capacity and for esthetic reasons.
Both copper and silver have wide spectrum antimicrobial properties. Some microorganisms are more sensitive to copper and some more sensitive to silver. The biocidal mechanism is similar in both cases, i.e. non-specific multisite kill mechanisms. Currently in the marketplace there are many “silver dressings” but only one “copper dressing” that have been cleared for clinical use by the FDA and European regulatory bodies. In regards to antimicrobial wound dressings, their antibacterial efficacy depends on several parameters, such as the exact silver or copper compound used, the concentration of the active agent, etc. Most of the silver dressings in the marketplace have been cleared for use many years ago, when the biocidal requirements by the regulatory bodies were lower. It is sure they would have been cleared today, as their antibacterial efficacy is not high. We have compared the biocidal potency of many silver dressings versus MedCu copper dressing using the well accepted quantitative test method – AATCC TM 100 – and found that copper dressings demonstrated much more potent efficacy than almost all silver dressings tested.
MedCu impregnates both layers of the wound dressings, the external nonwoven layer and the internal absorbent layer, with cuprous oxide microparticles. These microparticles are physically embedded in the polymeric matrix during the manufacturing process. These particles do not detach from the polymeric substrate, as demonstrated to the FDA and other regulatory bodies, but these particles serve as a reservoir of copper ions, which are constantly liberated in the presence of skin humidity and wound exudates.
The external wound dressing layer is a non-adherent layer that allows the passage of the wound exudates into the highly absorbent internal layer of the dressing. With the wound exudates bacteria present in the wound are also absorbed to a certain degree. The released copper ions reach a concentration that results in the killing of the bacteria, in the wound dressing itself and with time also in the tissue. We have demonstrated to the FDA that the wound dressing kills more than 4 logs of gram positive and gram negative bacteria immediately and the dressing preserves this capability also after being fully absorbed with wound exudates for 7 days.
As explained above in point 3, we have data for 7 days. Accordingly, the wound dressings are to be used for up to 7 days or until the wound is fully soaked with wound exudate.
The wound dressing can absorb ~ 8-10 times their own weight, depending on the SKU (dressing type). As long as the dressings have not reached their maximum absorbency capacity, the absorbed exudate (and bacteria) are entrapped in the dressing. The dressings are usually changed before reaching 7 days per single use in middle and highly exudating wounds. Nevertheless, we have repeatedly observed that a highly exudating wound will culminate in wetting and even greenish discoloration of the external paddings, but without harm to the skin at the wound edge. The explanation to this is that the dressing actually has two layers of bacterial killing properties: the inner one with higher concentration and the absorbent layer which still has cuprous oxide in bactericidal concentration. We rarely saw skin irritation and maceration even in highly exudating wounds. In addition, it is possible to change the outer padding without changing the copper dressing on the wound, just to receive more outer absorbent capacity and for esthetic reasons.
We have conducted the following safety studies using independent laboratories and GLP in accordance with the requirements for medical devices and passed all of them successfully:
- Acute systemic toxicity (Mice)
- Wound healing chronic toxicity study (Porcine)
- Skin irritation (Rabbits)
- Intracutaneous reactivity (Rabbits)
- Sensitization (Maximization) (Guinea pigs)
- Cytotoxicity (cell culture)
- Pyrogenicity test (Rabbits)
In addition, we successfully passed the exhaustive extractions, FTIR, GC-MS, ICP-OES, UPLC-MS test performed using GLP, demonstrating the high purity and safety of the dressing.
Based on the above extensive safety studies, MedCu wound dressings were cleared by the FDA and other regulatory bodies to be used clinically.
Currently the dressings are being used clinically in several countries for over 2 years, with hundreds of patients already being treated, without even one adverse reaction recorded.
In contrast to some silver dressings that can cause coloration, copper does not cause any argyria or any skin coloration. Copper oxide impregnated consumer products, such as pillowcases, diapers, medical textiles (sheets, blankets, etc.), socks, etc. are being used for more than 15 years by hundreds of thousands of individuals worldwide without any irritation reports, etc. This is the same exact technology used by MedCu by the sister company Cupron. On the contrary, these products have very good effects on the skin of those using them.
If you take human cells and expose them to a high concentration of copper ions they will also be killed. However, since copper is an essential mineral to us, humans have developed over the ages different mechanisms of “controlling” and “neutralizing” the copper ions, and delivering them where they are needed. There are many proteins in the body that their role is to capture the copper ions (e.g. albumins in the blood) and deliver them where needed, either into the cells themselves by copper pumps, etc. or from the gut to the liver, for example, where there they are “delivered” in a non-active configuration to the relevant tissues where the copper ions are needed. These proteins, some called chaperons, are found in the blood and intracellularly.